Accelerate global market reach
of global efforts
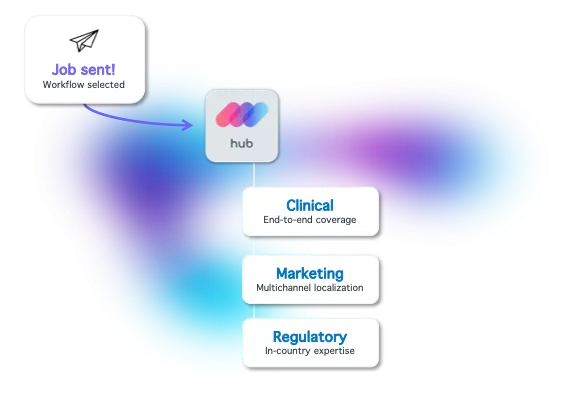
On-demand scalability across languages, cultures and regulatory bodies
Because the impact of technology is only as good as its relevance for specific applications, Linguamatics technology is developed for healthcare and life sciences specific use cases.
By blending advanced natural language processing, domain-specific terminology management and machine learning models, we enable rapid and accurate translation of critical documents, accelerate regulatory submissions and efficient knowledge-sharing, ultimately expediting the entire product development process from discovery to market launch.
Setting up the translation operation for global R&D efforts or trials is usually a tedious process. Linguamatics designed pre-built workflows and custom technology for research and clinical trials quick turnaround time. Linguamatics translate's AI engines, built and trained with R&D and clinical content, brings unmatched speed to the process.
Promotion & enablement localization is key for successful global market launches. Out-of-the-box workflows, simply integrated in the content ecosystem (DAM, PIM, CRM, CMS, etc.), save weeks in global roll-out...and weeks can mean millions.
The EUCTR challenged the regulatory timeline for local submissions, which mandates for certified translations to be handled a lot faster. Safety monitoring also requires a very short timeframe of submission and, in many markets and languages, it becomes critical to implement the right solution.